 DIY Builds
DIY Builds
 DIY Builds
DIY Builds

 Photo: mahdi chaghari
Photo: mahdi chaghari
These experiments establish that rats can communicate fear and induce specific odor fear learning via pheromone information.

You can run a 12 gauge wire up to 70 feet on a 15 amp circuit. That number drops to 50 feet if you run 12 gauge wire on a 20 amp circuit.
Read More »
To run electricity to a shed or outdoor building, start by planning out the the electrical cable routing. Next, add a GFCI outlet to the home's...
Read More »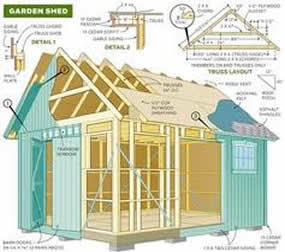
These are! They guide you every step of the way to complete your dream shed.
Learn More »
Florida has a large aquifer system that spans around 100,000 square miles and provides water for many large cities. The groundwater's very close to...
Read More »
Eight states have no personal income tax, including Alaska, Florida, Nevada, South Dakota, Tennessee, Texas, Washington, and Wyoming. ... The top...
Read More »
The double FISH protocol was established previously18. Briefly, full length Arc riboprobes conjugated to digoxigenin and H1a riboprobes conjugated to fluorescein were obtained using commercial transcription kits (Maxiscript) and RNA labeling mixes (Roche). Riboprobes were purified using RNA mini quickspin columns (Roche) and verified via agarose gel. Slides were thawed for 30 minutes at room temperature, fixed in 4% paraformaldehyde at 4 °C, bathed in acetic anhydride and acetone/methanol (Fisher Scientific), and treated with pre-hybridization buffer and hybridization buffer (Sigma-Aldrich) containing Arc and H1a probes. Slides were hybridized overnight in a 56 °C oven. All steps until this point were performed in the absence of RNAse. Slides were washed in a series of sodium citrate solutions followed by cleavage of any remaining single-stranded RNA using RNAse A. Endogenous peroxidases were quenched with H 2 O 2 and slides blocked with 5% sheep serum (Sigma-Aldrich). Arc riboprobe was detected with anti-digoxigenin-POD (Roche) and a TSA cyanine-3 substrate kit (Perkin Elmer). Following Arc detection slides were dipped in 2% H 2 O 2 solution to quench any residual HRP activity. H1a riboprobe was detected with anti-fluorescein-POD (Roche) and a TSA Fluorescein Tyramide substrate kit (Perkin Elmer). Nuclei were counterstained with DAPI (Sigma-Aldrich). Slides were coverslipped with Vectashield antifade medium (Vector Laboratories), sealed with clear nail polish, and kept at 4 °C before confocal microscopy scanning. Image Acquisition and Analysis: All slides were scanned in a Fluoview FV1000 confocal microscope (Olympus). All images were taken at 20X magnification. The photomultiplier tube assignments, confocal aperture size, and contrast remained constant for each slide. The z-stacks (optical thickness: 1.0 μm) were taken throughout the thickness of the section and were acquired from 3–4 slides for each animal. The mitral cell layer was analyzed in the olfactory bulbs, including the dorsolateral and ventral medial regions in the MOB. Layer II was analyzed in the PC, and the dense cell layer was analyzed in the OT. Images were analyzed from the center of each of the amygdala subdivisions. ImageJ software was used for counting cells in the scanned images. In all areas except the OBs total cell counting was done automatically for the DAPI stained nuclei; images were cropped to include only the area of analysis, transformed to binary images (black and white), and cells were counted using the “Analyze Particles” function in ImageJ. For the H1a+ and Arc+ cells, counting was done manually by checking 20% of the mid-range of the stack that comprised each cell. Average cell counts of Arc+ cells were divided by the average cell counts of H1a+ cells to compute a ratio of cells active to the conditioned odor versus cells active to the control odor for each animal.

Wood Sheds are the Standard in Durability Plastic sheds are flimsy in harsh weather and are prone to warping in very hot or cold conditions. Wood...
Read More »
“Use bars of Irish Spring soap for your deer problem and they'll go away,” Mrs. Poweska advised. “Just use a grater and shave the bars of soap into...
Read More »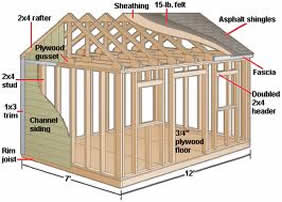
To study the role of NMDA receptors in the basolateral amygdala, a separate cohort underwent cannular implantations. Cannular surgeries were performed 1 week before the behavioral experiments. During surgeries, rats were anesthetized with isoflurane gas and secured in a stereotaxic apparatus. Two holes were drilled 2.5 mm posterior, and 4.9 mm bilateral relative to bregma for the BLA. Guide cannulae were inserted 7.8 mm ventral to the skull surface. Guide cannulae were secured by dental cement to two skull screws. The skin was sutured and the rats were returned to their cages for recovery. O+/S+ and pheromone molecule conditioned (O+/Ph) rats were infused with either saline or D-APV (5 mM; 1 µl) bilaterally into the BLAs 30 min before the conditioning experiments. Infusion tubing and cannular attachment were performed during habituation for animals to become acclimated to the attachment of the infusion tubing.

Agave Americana Agave Americana Blooms Once Every Hundred Years It's also known as a century plant because it only blooms once every 100 years...
Read More »
You can expect a fiberglass exterior door to last 50 to 100+ years, with an average of 70 years. Fiberglass does not rust or rot and is more...
Read More »

What Type of Fence Lasts the Longest? Chain-link fences with a galvanized finish that doesn't rust are the longest lasting fences. All other...
Read More »
The size of your shed determines the size of the skids you need. Pressure treated lumber is recommended and should last longer and support the...
Read More »